2017 Biomedical R&D and Industry Globalization Summit Forum
2017 Biomedical R&D and Industry Globalization Summit Forum
The world's first international adenovirus vector gene drug CMO platform has settled in
The press conference of Shenzhen Dapeng International Biotech Valley was held in Shenzhen

On June 6, 2017, the "2017 Biomedical R&D and Industry Globalization" Summit Forum and the world's first international adenovirus vector gene drug CMO platform settled in Shenzhen Dapeng International Biotech Valley. The press conference was held at the Wuzhou International Hotel in Shenzhen. Ada Yonat, the winner of the 2009 Nobel Prize in Chemistry, Wang Junzhi, the chief expert of biological product verification of the China Institute for Food and Drug Control, Wei Yuquan, an academician of the CAS Member, Luo Xielong, president of the China Pharmaceutical Enterprise Management Association, Wu You, deputy secretary general of Shenzhen Municipal Government, Qiu Xuan, secretary of the Party Leadership Group of the Shenzhen Science and Technology Innovation Commission, Wang Jingdong, secretary of the Party Working Committee of Dapeng New Area, and many other well-known scientists, entrepreneurs from famous universities and research institutions in the United States, the United Kingdom and China, as well as investors and representatives of government departments in the biomedical industry attended the conference, and witnessed the signing ceremony of the world's first adenovirus vector gene drug CMO international platform settling in the Baguang core start-up area of Shenzhen International Bio Valley.
Shenzhen Dapeng New Area and Shenzhen Saibainuo Gene Technology Co., Ltd. signed the "Shenzhen Dapeng New Area Saibainuo (International) Gene Life Science Park Project Strategic Cooperation Framework Agreement" at the meeting, marking the official settlement of the world's first adenovirus vector gene drug CMO international platform in Shenzhen Dapeng Baguang International Biotechnology Valley. During the meeting, a three-way signing ceremony was also held between the Sanozon CMO platform, Sequoia Cardiam Pharmaceutical Group and Meconodi Medical Technology Co., Ltd., and the signing ceremony between the Sanozon CMO platform and Hangzhou Yiyuan Biology Co., Ltd., to introduce the first class of biological bridging biological new drug "Maizaitong" (recombinant human fibroblast growth factor adenovirus) and the first class of biological new drug "Shengshengmei" (mutant glucokinase gene adenovirus) for the treatment of type II diabetes into the Sanozon CMO platform, and together with the first class of biological new drug "Jinzaitong", become the three pillar drugs of the Sanozon CMO platform. Shenzhen Saibainuo Gene Technology Co., Ltd. plans to promote the construction of the Sainosheng (International) Gene Life Science and Technology Park project in Dapeng New Area, Shenzhen in the next 3-5 years, to achieve the settlement of three or more "Class I" new drugs in Dapeng New Area, and jointly create a cluster of gene medicine industries.
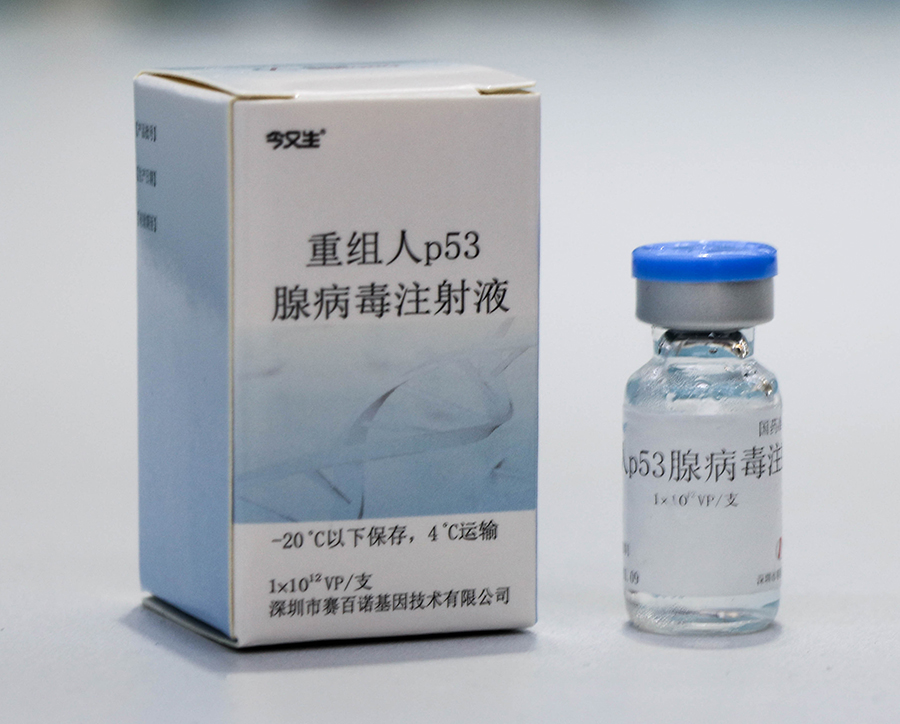
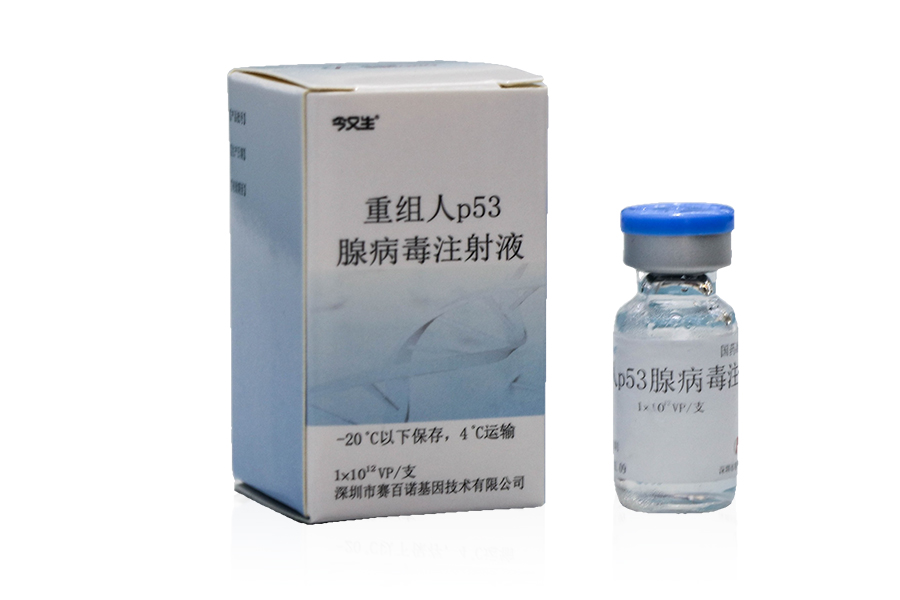
Sanosheng (Shenzhen) Gene Industry Development Co., Ltd., based on the creation of the world's first independently developed gene therapy anti-cancer drug "recombinant human p53 adenovirus injection" (also known as "Jinyousheng"), established the technical standards for large-scale production process and quality control of recombinant adenovirus products. In March 2003, it became the reference of China's Technical Guidelines for Human Gene Therapy Research and Preparation Quality Control, which was officially promulgated and implemented by the State Food and Drug Administration; And with the approval of China's CFDA, the full English version of the guiding principles was first published in the May 2004 issue of Blopharm International magazine for global reference. China has achieved a leap from laboratory to industrialization in the field of gene therapy, laying a leading position in the world of gene therapy.
Today's signing is in line with Shenzhen's new round of technological innovation strategic layout, "said Qiu, Secretary of the Party Group of the Municipal Science and Technology Innovation Commission. Xu Wei, the chairman of Sinolife, stated that Dapeng New Area is the last "blue ocean" of Shenzhen's biological life and health industry. There is Qianhai in the west and Baoguang in the east. The core startup area of Shenzhen International Bio Valley will become a new economic growth pole in Shenzhen, focusing on the development of eight industries including life information, biomedical engineering, biomedicine and high-end medical care, life and health services, biological resource development, and biological environmental protection and manufacturing. The establishment of the world's first adenovirus vector gene drug CMO international platform in the core launch area of Baoguang will undoubtedly drive a new wave of biopharmaceutical development in the International Biotech Valley.
Ada Yonat, the winner of the 2009 Nobel Prize in Chemistry, gave a brilliant speech on "The New Generation of Environmentally Friendly Antibiotics" at the conference. After the speech, in an interview with reporters, she talked about the Sinovac CMO platform and Shenzhen Dapeng Baguang International Biotechnology Valley, saying, "If we can find a research topic that both parties are interested in, I am very interested in collaborating with Shenzhen and Dapeng. I particularly hope to jointly develop new antibiotic drugs
Wang Junzhi, Chief Expert of Biopharmaceutical Testing at the China National Institute for Food and Drug Control, presented a wealth of detailed data at the meeting, introducing the current trends and research priorities in the development of biopharmaceuticals both domestically and internationally. He also discussed the latest developments in China's major new drug creation and biopharmaceutical development, and affirmed the formation of Sinovac's CMO platform and WHO's recognition of Chinese companies' path towards industrialization of adenovirus vector gene drugs.
Wei Yuquan, an academician of the CAS Member, also said that the growth rate of China's pharmaceutical market in recent years is higher than the GDP growth rate, which is mainly related to the aging of the population, the acceleration of urbanization and the improvement of major treatment methods, not due to the industrial structure. At present, although there are numerous Chinese pharmaceutical companies, their research and development capabilities are generally weaker than those of foreign pharmaceutical companies, and their industrial transformation capabilities are insufficient. But in the field of gene therapy, especially in the direction of adenovirus vector drugs, China is in an advantageous position internationally in terms of basic research, clinical research, and industrial transformation.
Guo Yunpei, President of the China Pharmaceutical Enterprise Management Association, pointed out in his speech that in the past 10 years, the Chinese pharmaceutical industry has maintained an average annual growth rate of 15%, mainly due to the health needs and medical insurance coverage released by the improvement of people's living standards. China has become one of the world's major pharmaceutical countries, but it is only a major producer of generic drugs and active pharmaceutical ingredients. In 2015, the global market for innovative drugs was close to 600 billion US dollars, with the Chinese market accounting for less than 10 billion US dollars. Among them, 19 innovative products approved for the first time in China contributed less than 500 million US dollars, and their sales were limited to the Chinese market. He believes that China still has a long way to go before becoming a major innovative pharmaceutical country and a world pharmaceutical powerhouse. At present, China is only in the third tier of the global pharmaceutical innovation landscape, contributing only 4% to global pharmaceutical innovation annually, while the United States in the first tier is about 50%. Biopharmaceuticals will be a breakthrough point for China's pharmaceutical industry to achieve a turning point overtaking, and Shenzhen is seizing a new high ground in biopharmaceuticals in a new round of technological innovation strategic layout. President Guo summarized in the meeting that "the development prospects of China's biopharmaceutical industry are limitless
The President of the China Pharmaceutical Enterprise Management Association, Luo Xielong, highly praised the conference, stating that the report presented at the conference has significant guiding significance for the research and industrialization of Chinese medicine, especially biopharmaceuticals, and has pointed out the direction for the development of biopharmaceuticals. As a successful case, the Sinopharm CMO platform is a new R&D innovation model that illustrates how Chinese pharmaceutical companies can go global through independent innovation, R&D industrialization, and government policy support to achieve internationalization of China's pharmaceutical industry. It is bound to bring international parallel development of adenovirus based gene drugs to Dapeng International Biotech Valley, thereby transforming China from a pharmaceutical powerhouse to a pharmaceutical powerhouse.