Commemorating the 40th anniversary of p53 discovery: yesterday, today, tomorrow!
2019 marks the 40th anniversary of the discovery of p53. To commemorate major scientific discoveries, pay tribute to the masters of molecular cell biology, exchange the latest achievements in the field of tumor research, and look forward to the application prospects of p53 family in cancer treatment, the Journal of Molecular Cell Biology (JMCB) held the JMCBSymposium 2019: The Legend of p53 vs. Cancer in Hangzhou from May 10-12, 2019. Professor Hua Lu, Deputy Editor in Chief of JMCB and from Tulane University in the United States, reviewed and organized the 40 year history of p53 since its discovery at the opening ceremony.

In 1979, David P. Lane from Cancer Research UK and Arnie Levine from Princeton University in the United States first discovered the existence of the p53 gene. These researchers may not have expected that their findings would usher in a new era of modern cancer research and treatment.

In the first 10 years, p53 was considered an oncogene that could induce tumor production and was not given much attention. It was not until 1989 that Bert Vogelstein, a molecular biologist at Johns Hopkins School of Medicine in the United States, finally found the correct p53 gene, wild-type p53, and people discovered that p53 is a powerful tumor suppressor gene that plays a wide range of roles in the human body. This is a milestone event in p53 and cancer research, and from then on, p53 research has entered the fast lane in the right direction.

In 1993, p53 was named the star molecule of that year by the journal Science. Until now, international academic conferences with p53 as the theme have never been interrupted.

In October 2003, China passed the p53 therapy. The design concept of "recombinant human p53 adenovirus injection" is based on using human adenovirus type 5 as a vector to introduce the wild-type p53 gene into the tumor site and exert therapeutic effects. This is the world's first approved p53 gene therapy drug, marking the transition of p53 research from theory to clinical research and application, and opening the curtain for gene therapy research and development.
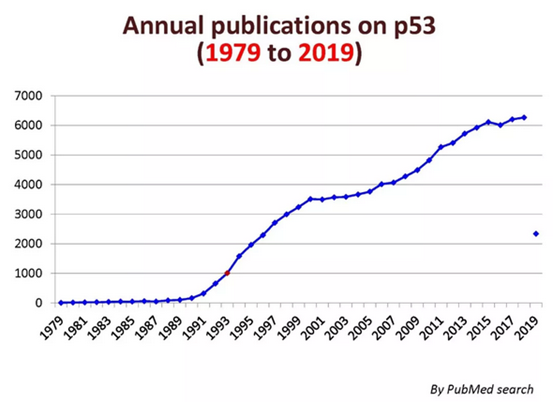
Among the tens of thousands of genes contained in the human genome, it is the most thoroughly studied. P53 has become a popular target in the development of anti-tumor gene therapy drugs. In the Biomedical Literature Database (PubMed) of the National Center for Biomedical Information in the United States, research literature on it is steadily increasing every year.
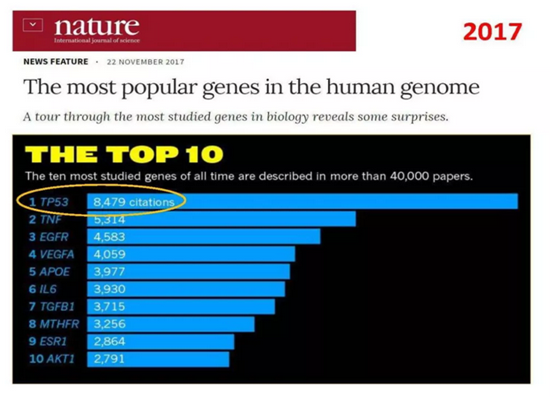
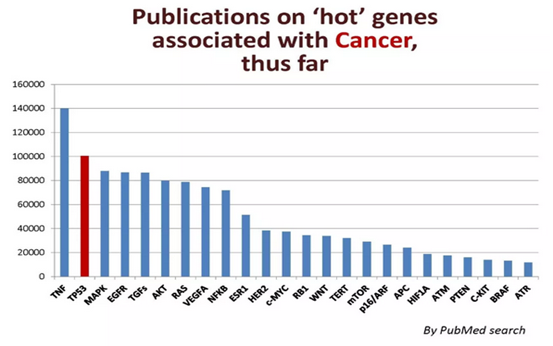
In the "The Most Popular Genes in the Human Genome" published by Nature News, a list of the "hottest" genes in human genetic research has been compiled, and the tumor suppressor gene TP53 is undoubtedly a star gene. TP53 gene, as the first tumor suppressor gene on the list, is one of the earliest discovered tumor suppressor genes and plays a very important role in inhibiting tumor cell growth, DNA repair, and programmed cell death. It is called the "guardian of the genome". More than 50% of human malignant tumors, including common liver cancer, breast cancer, bladder cancer cancer, stomach cancer, colon cancer, prostate cancer, soft tissue sarcoma, ovarian cancer, brain tumor, lymphocyte tumor, esophageal cancer, lung cancer, osteosarcoma, etc., are all related to P53 gene mutations.

In the Spring Festival of 2018, "Human Gene Therapy" issued a special issue summarizing and reporting on the clinical application of the world's first gene therapy for cancer for 12 years, titled "The First Approved GeneTherapy Product for Cancer Ad-p53 (Genicine): 12 Years in the Clinic".
From its approval in 2004 to 2016, Shenzhen Saibainuo Gene Technology Co., Ltd. produced a total of 41 batches, totaling 169571 units (1.0x1012 virus particles per unit), all of which met the QC/QA standards of the China Food and Drug Administration. More than 30000 patients, including nearly 10% of international patients from over 50 countries, have received treatment alone or in combination with radiotherapy, chemotherapy, hyperthermia, and other multiple therapies. Overall, in various clinical applications and studies, the use of modern treatment has achieved a complete response rate (CR) of 30-40%, a partial response rate (PR) of 50-60%, and an overall response rate (CR+PR) of 90-96%.
Experts at home and abroad have published more than 200 clinical documents, and studied the treatment of cancer with "recombinant human p53 adenovirus injection" alone or in combination with other therapies. The types of tumors involve head and neck cancer, breast cancer, lung cancer, cervical cancer, ovarian cancer, liver cancer, pancreatic cancer, esophageal cancer and advanced colon cancer. The "recombinant human p53 adenovirus injection" in various clinical literature resulted in an average increase of 25.77% in the remission rate of various tumor types.
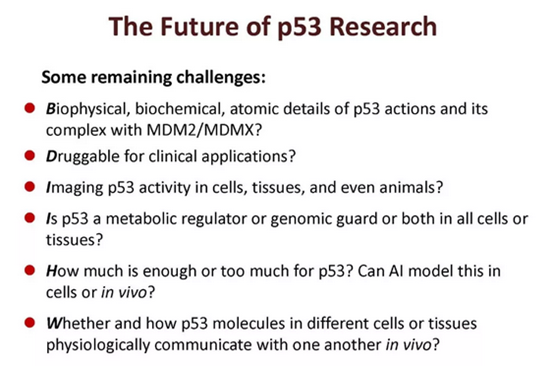
With the deepening of research on p53, it has been discovered that it is not only present in the field of cancer, but also in other diseases and physiological processes. These discoveries make him the most familiar stranger with multiple facets.