Media reports
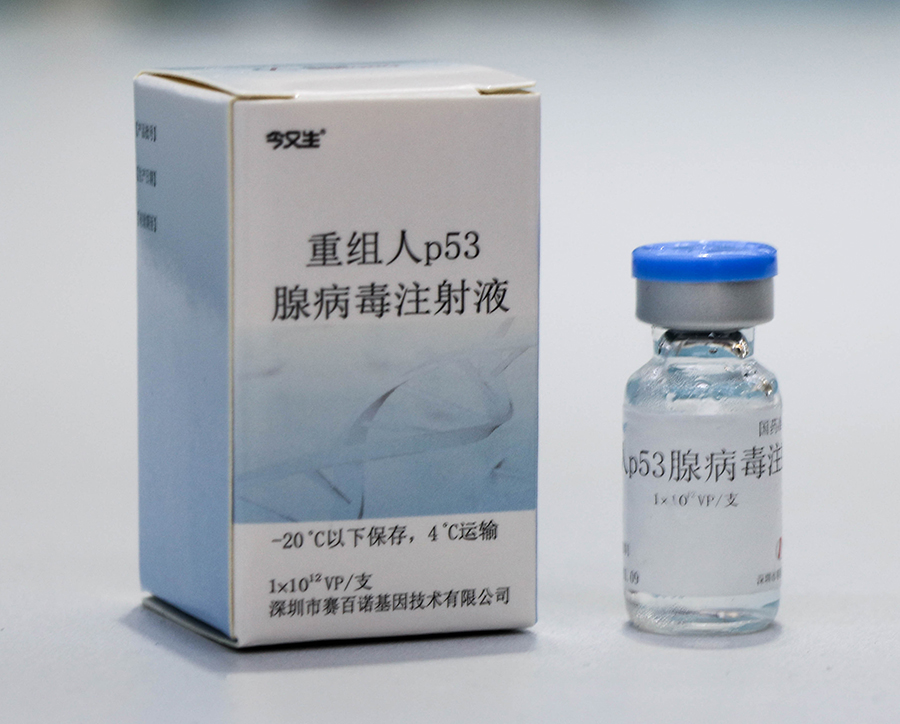
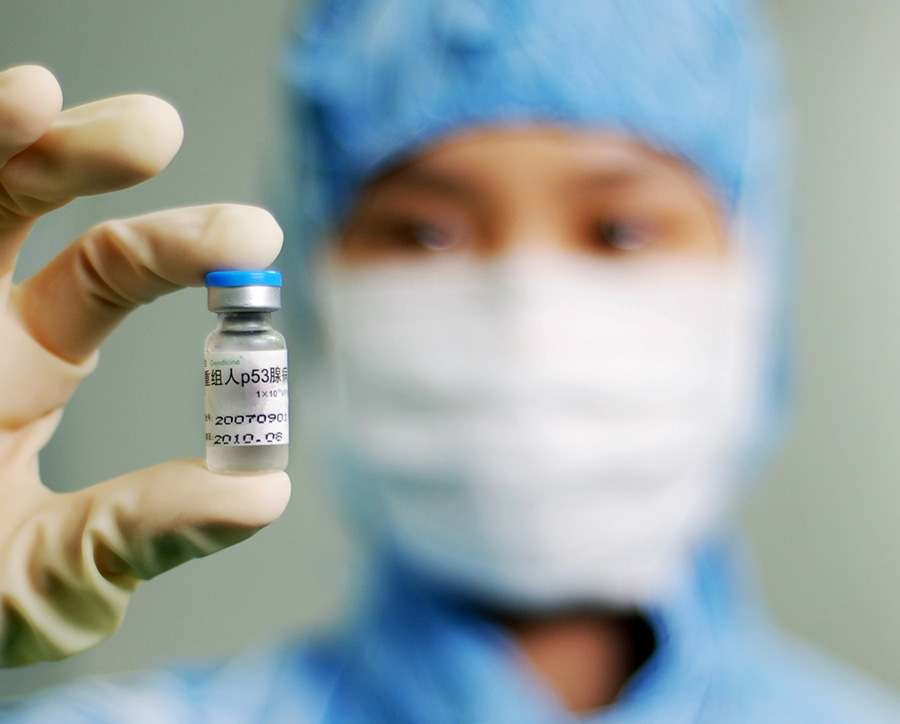
In October 2003, *********** gene therapy product "Jin You Sheng" (recombinant human p53 adenovirus injection) was successfully developed and officially approved by the China National Food and Drug Administration to be launched on the market. The launch of "Jin Re Sheng" has attracted widespread attention worldwide. Thousands of magazines, newspapers, websites, radio, and television outlets have focused on the theme of 'rebirth today', providing continuous coverage and commentary from different perspectives. The success of "Born Now" represents a solid step forward for humanity in the research, development, and industrialization of gene therapy, and a decisive achievement. It is hailed as a "new milestone in gene research and biotechnology, which will drive the development of the entire gene therapy research and industry, have a profound impact on the world's healthcare system, and make important contributions to human health

On March 1, 2004, People's Daily reported that the world's first gene therapy drug was officially approved for marketing in China

On September 9, 2006, CCTV News reported with the headline:
Participate in standard setting and gain market opportunities

Time Magazine, October 2006:
These inventions in the field of biotechnology will change our way of life and establish China's position as a scientific superpower
The approval of the listing of 'Jin You Sheng' will push China to enter the forefront of this (gene therapy) field