Analysis of the Development of Virus Carrier Industry
Author: Miao Xianfeng
(This article is published under the authorization of WeChat official account "Firestone link" (ID: firestone link), and its copyright belongs to the original author. If it is reproduced, please contact the original author.)
On August 30, 2017, Novartis Kymriah was approved, marking the beginning of gene therapy. Gene therapy may become a key research and development direction for innovative drugs in the next decade, following antibody drugs. As a key component of treatment, viral vectors are crucial for gene therapy.
1、 Introduction to viral vectors
In 1990, Dr. William French Anderson successfully treated a 4-year-old girl with severe combined immunodeficiency using gene therapy for the first time, marking the beginning of gene therapy research and development. Humanity has found the "hand of God" to overcome the disease.
On August 30, 2017, Novartis Kymriah was approved, becoming the first gene therapy drug approved by the US FDA, thus opening up the curtain of gene therapy and bringing new opportunities for rare disease treatment. Gene therapy may become a key research and development direction for innovative drugs in the next decade, following antibody drugs.
1. Gene therapy mechanism
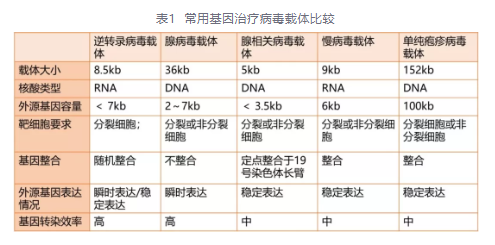
Gene therapy involves introducing a genetic information into diseased cells or organs to correct defects or abnormalities (mutations, abnormal expression, etc.), in which viral vectors are crucial. The commonly used viral vectors for gene therapy currently include retroviruses (RV), adeno-associated viruses (AAV), and lentiviruses (LV).
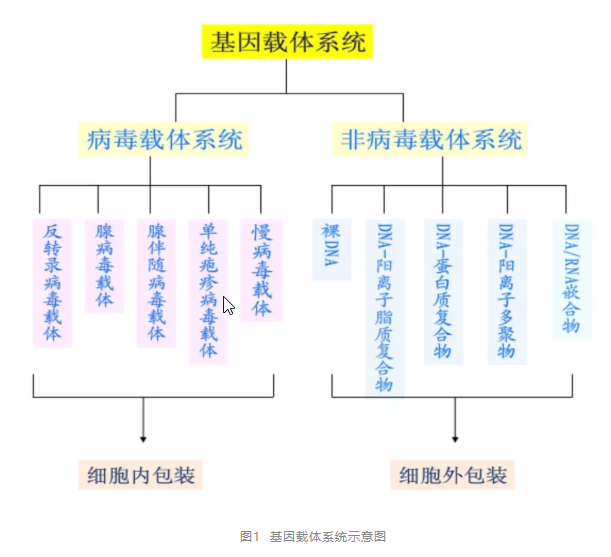
Gene therapy vectors can be divided into two categories: (1) viral vectors, mainly including lentivirus, adenovirus, retrovirus, adenovirus, etc. (2) Non viral vectors mainly include naked DNA, liposomes, nanocarriers, etc.
2. Some commonly used viral vectors
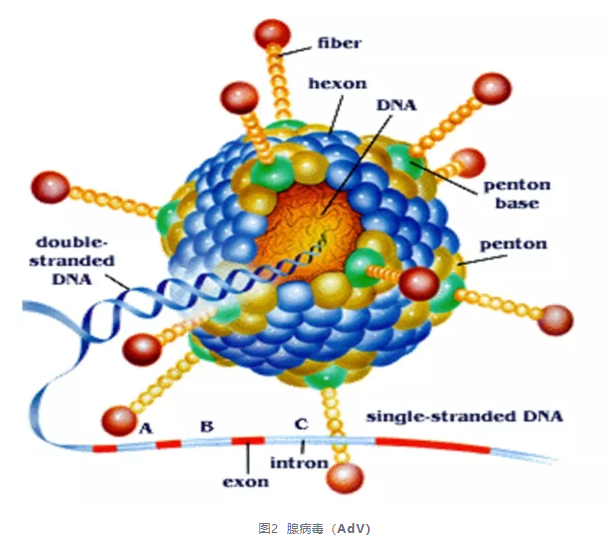
Adenovirus (AdV) is a large molecule (36 kb) double stranded non enveloped DNA virus. Host cells have a wide range and can infect both dividing and non dividing terminally differentiated cells, such as neurons.
Advantages: (1) High gene transfer efficiency and safety for humans; (2) Wide host range; (3) Gene transduction is not related to cell division; (4) The recombinant adenovirus can be absorbed through the intestinal tract by oral administration, or inhaled by spray or intratracheal instillation; (5) The adenovirus vector has a large capacity and can insert 7.5kb exogenous genes.
Disadvantages: (1) Cannot be integrated into the genomic DNA of target cells; Cells that divide and proliferate rapidly have an increased chance of losing their recombinant viral vectors during division, and their expression time is relatively short. (2) The host's immune response leads to transient expression of adenovirus vectors; (3) There are two possible stages that may produce replicating adenovirus; (4) Poor targeting ability.
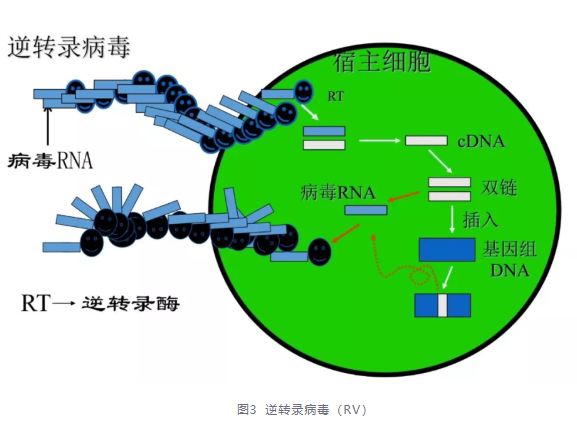
Retrovirus (RV): A single stranded RNA virus that can efficiently infect various types of cells. It can randomly insert exogenous genes and stably integrate them into the host cell genome for sustained expression. Among them, the gamma retroviral vector was the earliest modified and widely used in gene therapy, achieving significant success.
Defects: (1) Can only transfect cells in a proliferative state; (2) The foreign genes carried should not be too large; (3) Infection depends on the restriction of target cell surface receptors; (4) In theory, it does not spread to other cells, but in some cases it can cause outbreaks of wild-type viruses; (5) There is a possibility of causing cellular carcinogenesis; (6) Retroviruses cannot tolerate purification and concentration processes;
To address the shortcomings of current viral vectors, scientists have never stopped advancing in the research of new viral vectors.
In December 2017, at the Viviana Gradinaru Laboratory of the California Institute of Technology, a new variant of the adeno-associated virus (AAV) vector was developed: an AAV-PHP.EB vector that can effectively transport genetic material to the blood-brain barrier; Another type of AAV-PHP S is effectively absorbed by peripheral neurons residing outside the brain and spinal cord, such as those that sense pain and regulate heart rate, breathing, and digestion.
In May 2018, the National Nanoscience Center of the Chinese Academy of Sciences made research achievements in the light controlled release nano delivery system and non viral nano carrier delivery based on the gold nanoparticles liposome system. They have developed a series of non viral nanocarriers that can efficiently deliver CRISPR/Cas9 systems into the body, providing new avenues for expanding the application of this powerful gene editing technology in life sciences and clinical fields.
In December 2018, Poseida announced the latest non viral vector BCMA CAR-T ASH
2、 Layout of domestic virus vector platforms
In order to seize the development opportunities of the gene therapy industry and provide professional service platforms for gene therapy enterprises, the domestic virus vector professional platform has been planned since 2017 and gradually implemented.
1. Sinogene (Shenzhen) Gene Industry Group Virus Vector Gene Drug CDMO Platform
On May 3, 2018, the industrialized CDMO platform for adenovirus vectors with GMP system in ****** has passed the inspection and approval of Guangdong Provincial Medical Products Administration. Sinogene (Shenzhen) Gene Industry Group's CDMO platform for viral vector gene drugs, based on its own core intellectual property rights, professional talent team, unique process research and development capabilities, and large-scale production capabilities, provides integrated services of "contract research and development+customized production" for key links from preclinical research and development to commercial production for global viral vector gene drug developers.
2. Heyuan Shanghai virus vector CDMO platform
On March 5, 2019, Heyuan Shanghai opened its CDMO platform for virus vectors. This platform is the world's first GMP virus production platform based on disposable technology, built by Heyuan Shanghai with hundreds of millions of yuan and in collaboration with GE Healthcare, covering an area of nearly 4500 square meters. The GMP workshop adopts a 50 L disposable reactor equipment, which is at the forefront internationally.
At present, the platform has cooperated with multiple gene therapy or cell therapy drug companies to apply for CDMO projects, including adenovirus, lentivirus, adenovirus, poxvirus, naked plasmids and other related products. It provides one-stop services from small-scale, pilot, clinical application to GMP production, meeting the development needs of gene therapy and CAR-T cell therapy drug customers at different stages at home and abroad.
3. Nanjing Kingsray Biotechnology and Merck have launched a strategic cooperation in the field of cell gene therapy
On March 20, 2019, Nanjing Kingsray Biotechnology, a biopharmaceutical CDMO company, and Merck, a technology company, jointly announced the first comprehensive strategic cooperation in the field of cell gene therapy, jointly promoting the industrialization process of cell immunity and gene therapy industry.
According to the agreement, Merck will provide 360 degree comprehensive services to Kingsray in plasmid virus production, including product and process technology, factory design consulting, quality system establishment, GMP compliance consulting, personnel training, etc., to support Kingsray's plasmid and virus vector production service platform construction and internationalization strategy. The two companies will engage in comprehensive cooperation in plasmid virus quality system and GMP compliance, process and plant design, and market promotion.
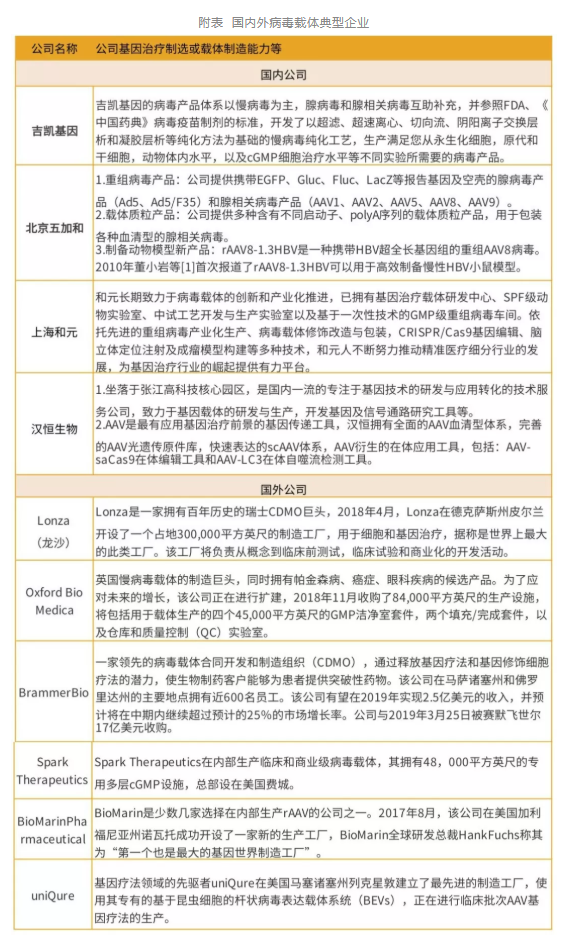
3、 Typical enterprises of virus vectors at home and abroad
Typical domestic virus carrier companies include Jikai Gene, Beijing Wujiahe, Shanghai Heyuan and Hanheng Biotechnology, etc; Typical foreign viral vector companies include Lonza, Oxford Bio Medica, Brammer Bio, uniQure, Spark Therapeutics, and Bio Marin Pharmaceutical.
reference:
[1] Dong Xiaoyan, Wei Chijie, Wang Gang, etc Preparation of a mouse model of sustained hepatitis B virus infection by in vivo transduction of highly hepatotropic recombinant adenovirus type 8 [J] Journal of Virology, 2010 (6): 425-431