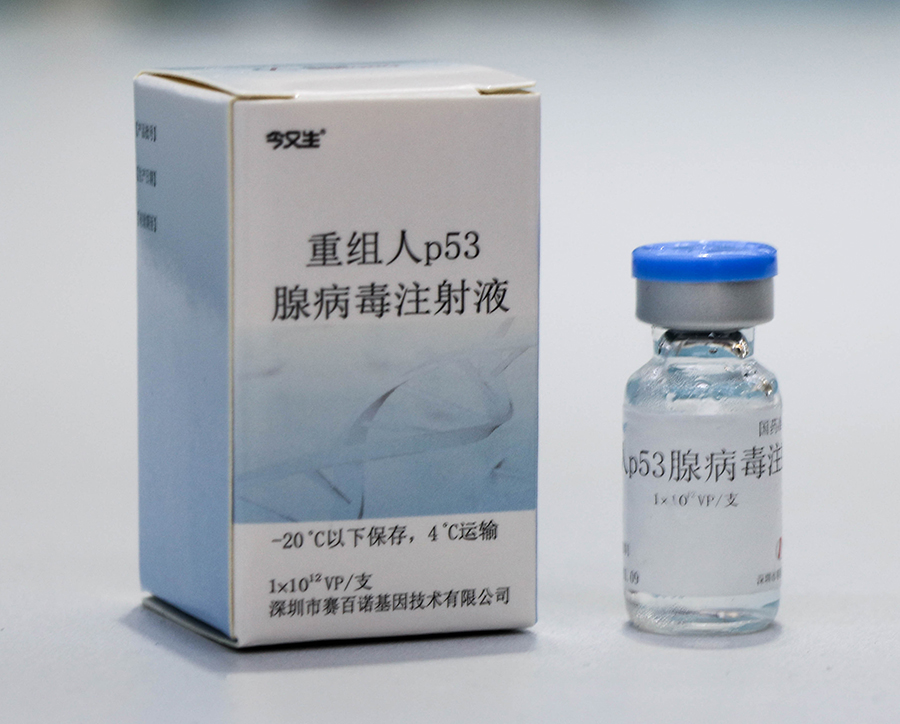
Market advantage
Scinosen has solved the worldwide problem of large-scale production of virus-based gene drugs under GMP conditions for pilot trials and GMP certification, and has become a global provider of viral vector gene drugs industrialization services with great influence.
1. The world's first adenovirus vector gene drug CDMO platform, with well-equipped GMP workshop and 6,500 square meters of plant, quality testing space of 700 square meters, its industrialization scale is the largest in the world.
2. The core R & D and industrialization team, with more than 10 years of adenovirus vector gene drug large-scale production technology experience, master the world's leading non-patented key technology.
3. In June 2016, the Chinese government issued the "Pilot Plan for the Holder System of Drug Marketing Authorization", which allows enterprises with drug marketing authorization to produce their own products or entrust product production to manufacturers with GMP conditions.
4. Its three core technologies have the world's leading level.