Scinosen CDMO Platform
Introduction of Scinosen genetic drug CDMO Platform
Scinosen gene scientists team and industrialization core team - is the world's first gene drug (Gendicine) founder, established a set of recombinant adenovirus biological products large-scale production process technical standards and quality control technical standards. This standard has established China's leading position in the world in the field of large-scale industrialization of adenovirus gene drugs. It has established drug production quality management norms that comply with China's NMPA, the US FDA and the EU EMA.
Based on the team's more than 20 years of accumulation in the industry, Scinosen focuses on providing professional viral vector gene drug CDMO services for the world, and is committed to promoting the development of new gene drugs to make its contribution. The team will create more than expected returns for investors in Scinosen gene drug CDMO platform!
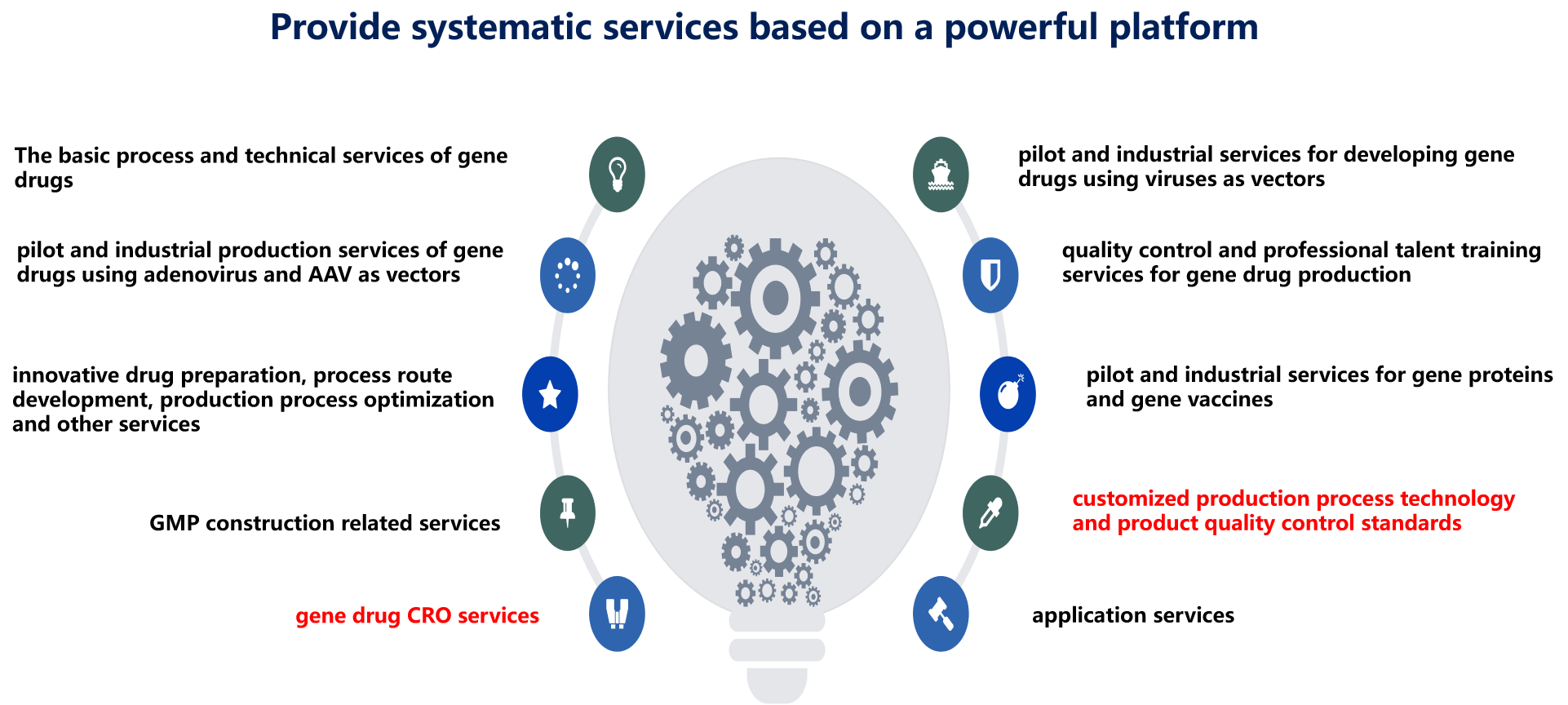
The core value of Scinosen CDMO platform is that it has a mature production management and quality control system unique to the field of gene drug industrialization, and it has a strong team of gene drug research and development and industrialization. The team will exert its unparalleled research and development ability to optimize product preparation process for customers' gene drug projects under the conditions of cGMP. And complete the customer's custom research and development commission. Scinosen CDMO platform can provide gene drug developers with the research and development and optimization of the technological process required in the production of innovative drugs, gene drug construction scheme and formulation development, pilot production services, and gradually provide customized production services from kg to ton level on the basis of the above research and development services.
Scinosen CDMO platform combines its high-tech value-added process R&D capabilities with large-scale production capabilities, and can effectively connect the entire supply chain system of gene drug developers' R&D, procurement and production through clinical trial production and commercial production supply modes, and export services with high value-added technology and production capacity with high industrial threshold. To provide customers with innovative process research and development and large-scale production services.
Scinosen CDMO platform, relying on its core intellectual property rights and a strong team of professionals, as well as years of industrialization system and experience, wholeheartedly provides the following high-quality services for global gene drug research and development institutions and enterprises:
【 Extended Reading 】
Characteristics and Differences of CRO, CMO, CDMO:
· The core of drug development (Research) lies in the technical personnel
· The core of drug manufacturing lies in the process and production capacity
· CMO who intervenes in production during the development phase and collaborates with customers earlier and more deeply
Compared to CMO, CDMO has stronger technical strength, closer customer relationships, stronger project acquisition and reserve capabilities, and more certain future growth potential.