About GeneTherapy
1. What is gene therapy?
Gene therapy is a therapeutic program that introduces normal genes or genes with therapeutic effects into the human body in a certain way to correct or supplement diseases (such as cancer, genetic diseases, diabetes, etc.) caused by gene defects and abnormalities.
In a broad sense, any therapeutic method that involves introducing exogenous genes (DNA or RNA) and exerting corresponding functions belongs to gene therapy.
In August 2018, the FDA and NIH jointly published an article in NEJM stating that there is insufficient evidence to prove that gene therapy poses special and unpredictable safety risks and does not require regulatory measures different from other treatment methods.
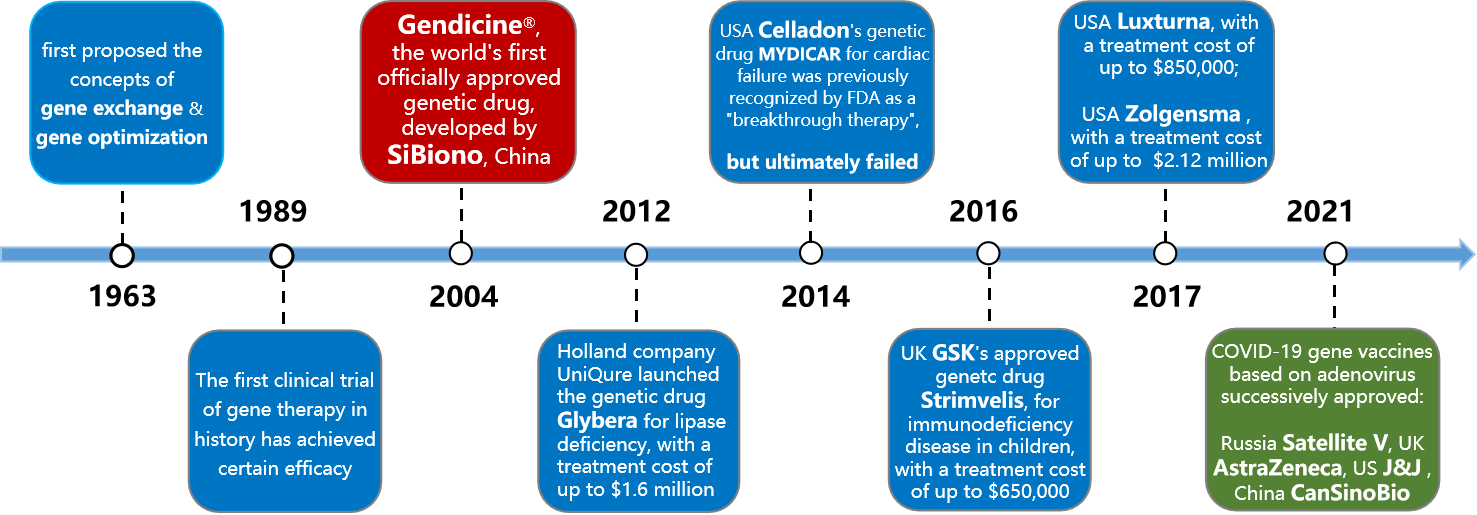
2. Milestones of gene therapy
3. The current development status of gene therapy:
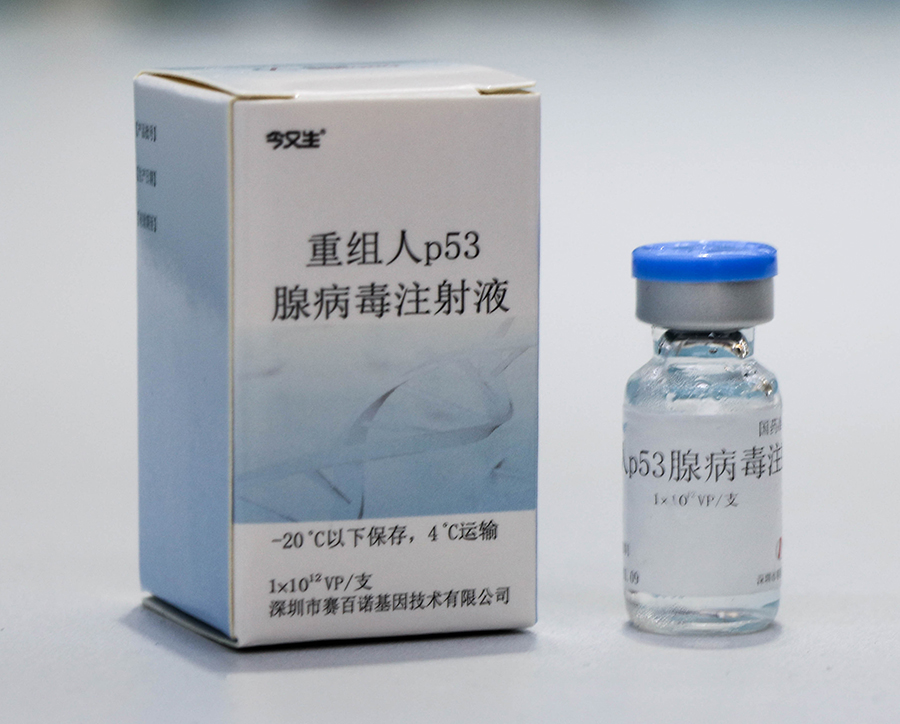
In 2003, Gendicine was approved in China as the first gene therapy drug for humans, marking a milestone achievement in the field of human genetics.
In 2012, Glybera, the first gene therapy drug in Europe, was approved, making it the second gene therapy drug approved for marketing in humans. This further promoted the development of gene therapy and was another milestone in the field of gene therapy;
In January 2018, Science magazine published an article titled "Gene therapy comes of age", which believed that gene therapy has ushered in a new era and once again aroused widespread attention from the pharmaceutical industry to gene therapy drugs. Large multinational pharmaceutical companies have also begun to make substantial investments in gene therapy research and development, and the research and development of gene therapy drugs has entered a rapid development stage.
At present, it seems that biological therapy technologies represented by gene and cell therapy will usher in the third revolution in the field of medicine.